Supramolecular Organometallic Chemistry in Separations and Catalysis
A project supported by the U. S. Department of Energy
Principal Investigator: Thomas B. Rauchfuss
Matthias Stein, Max Planck Institute for Dynamics, MagdeburgInternational
Ken Sakai, University of Kyushu
Coworkers:
Wan-Yi (Amy) Chu, BS National Tawain University
Elizabeth Kahle, BS University of of Illinois, Urbana-Champaign
Brian Lang, undergraduate researcher, University of of Illinois, Urbana-Champaign
Cassaday Richers, PhD University of of Illinois, Urbana-Champaign
Andrey Tregubov, PhD University of New South Wales
Edmund Tse, BS University of Virginia
Overview.
In this project we apply organometallic chemistry to basic issues related to sustainability, i.e. energy processing and environmental remediation. The coordination chemistry of simple unfunctionalized ligands is mature, and but complexes of functional ligands is filled with opportunities, inspired by supramolecular chemistry, organocatalysis, and nanoscience. Molecules are larger than before and expectations for selectivity and green-ness are high. Synthesis plays a major role guided by in situ methods of analysis.
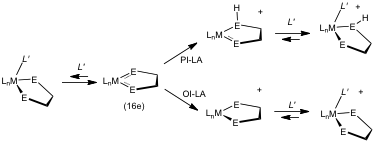
- Induced Lewis Acidity. The coordination of substrates is the basis of catalysis by metals, and methods to induce this binding or make it more selective are key goals. We have been examining two ways to induce binding. In the first, proton-enhanced Lewis acidity (PI-LA), ligands are protonated causing the metal to become highly Lewis acidic. An example of this behavior is the protonation of amido ligands in 16e iridium complexes:
In Oxidation-induced Lewis Acidity OI-LA), a ligand is oxidized, causing the metal to become highly Lewis acidic. For example, amidophenolates of Ir(III) are inert, but upon oxidation, these complexes bind a range of weakly basic substrates such as H2 and CO.
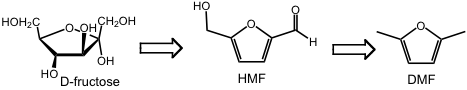
- Liquid Fuels from Biomass. Biomass is the world’s the largest supply of renewable fuels, representing solar energy stored in the form of organic compounds. Unlike petroleum, lignocellulose is a solid and is highly oxygenated. Technologies must be invented for conversion of the sugar and lignins into liquid fuels, we predict that metal-based catalysis will be part of the solutions. Illustrative of our efforts is the conversion of fructose to dimethylfuran (DMF) using formic acid as a deoxygenation agent.
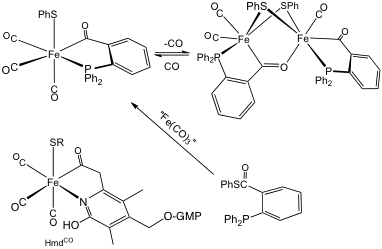
- Enzyme-Inspired Organometallics. Nature makes good use of organometallic chemistry for transformations of the energy-relevant substrates CO and H2. For example, an iron acyl thiolate center defines the active site of the enzyme Hmd (methylenetetrahydromethanopterin dehydrogenase, see bottom left image in figure). This enzyme catalyzes the heterolytic activation of H2 to effect reduction of a carbocationic substrate. Our progress in preparing models, indicated below, is highly versatile since the thioester can be generated in many forms.
- Non-innocent Ligands in Catalysis. Non-innocent describes ligands that undergo redox at potentials similar to those of the attached metals. Thus, both the metal and the ligand can undergo redox. Nature uses such ligands extensively, for example in O2 activation. Coordination chemists have been interested in them for decades, but surprisingly little work has been aimed at their use in catalysis. We have developed a family of half-sandwich complexes of non-innocent ligands for use in activation of hydrogen and related small molecules. The ligands are derived from 2-aminophenol, which is a flexible scaffold.
- Second Coordination Sphere Control with 2-Pyridones. We are examining the role of 2-hydroxypyridines (2-pyridones) in catalysis and coordination chemistry. Pyridines are well known as ligands, and the presence of the 2-hydroxy group presents an easy and versatile method for influencing the second coordination sphere of the associated complex. We are also studying the species 2-hydroxypyridine-6-acetic acid, an analogue of cofactor GP, which is involved in the methanogenesis pathway. A family of coordination complexes have been prepared, and the main focus is on structural models for biological H2-transfer catalysts.
- Metal-Amine-Hydrides as Prototype Catalysts for Fuel
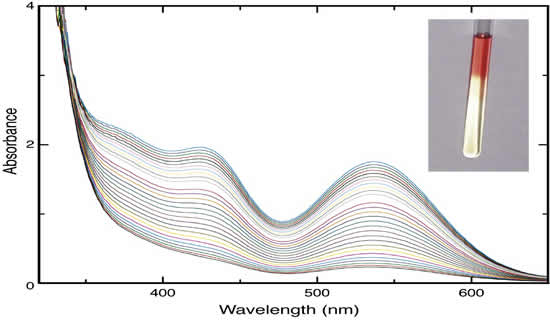
Cells. The very simple reaction O2 + H2 is both exothermic and slow in the absence of catalysts. We suggest that by elucidating pathways to accelerating this reaction, one might learn information that would inspire the design of new catalysts for fuel cells. Our main discovery is that Cp*Ir(NR2)(NH2R)H are excellent catalysts for this reaction. A key aspect of the catalysis is the participation of the N-H center in the hydrogenation of the dioxygen (See Figure).
Figure: Optical spectra for the hydrogenation of O2 by Cp*IrH(TsDPEN).
- Container molecules. Our container project is premised,
broadly speaking, on the expectation that geometrically rigid, kinetically
robust container molecules will increasingly become part of our technological
culture. Machines of the future will rely on these molecules for plumbing
and packaging. In this project we are preparing large organometallic cage
compounds based on CN-linked multimetallic arrays. Well defined, kinetically
robust, container molecules are difficult to prepare. Synthetic chemists
are expected to elucidate design rules that will guide this effort. And
simply being large is not enough, the molecules must be smart (functional)
and in our hands at least, soluble. We discovered a templated method to induce
condensation of CpCo(CN)3- and Cp*Ru(MeCN)3+ to
quantitatively produce the organometallic box [CpCo(CN)3]4[Cp*Ru]4:
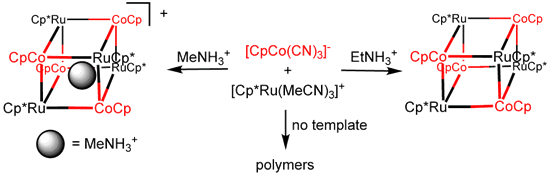
This species display a pronounced affinity for Cs+ even in the presence of substantial excess of K+. 137Cs+ in the presence of excess K+ presents an environmental problem associated with clean-up of nuclear wastes.
Representative Recent Publications
"Dioxygen and Hydrogen Peroxide Reduction with Hemocyanin Model Complexes", M. A.. Thorseth, C. S. Letko, T. B. Rauchfuss, A. A. Gewirth, Inorg. Chem. 2011, 50, 6158-6161.
"Coordination Chemistry of the Soft Chiral Lewis Acid [Cp*Ir(TsDPEN)]", C. S. Letko, Z. H. Heiden, T. B. Rauchfuss, Inorg. Chem. 2011, 50, 5558-5566.
"Activation and Deactivation of Cp*Ir(TsDPEN) Hydrogenation Catalysts in Water" C. S. Letko, Z. M. Heiden, T. B. Rauchfuss, Eur. J. Inorg. Chem. 2009, 4927-4930.
"Oxidative Addition of Thioesters to Fe(0): Active-Site Models For Hmd, Nature’s Third Hydrogenase" A. M. Royer, T. B. Rauchfuss, D. L. Gray, Organometallics 2009, 28, 3618-3620.
"Proton-Assisted Activation of Dihydrogen: Mechanistic Aspects of Proton-Catalyzed Addition of H2 to Ru and Ir Amido Complexes," Z. M.Heiden, T. B. Rauchfuss, J. Am Chem. Soc. 2009, 131, 3593-3600.
"Redox-Activation of Alkene Ligands in Platinum Complexes with Non-innocent Ligands," J. L.Boyer, T. R. Cundari, N. J. DeYonker, T. B. Rauchfuss, S. R. Wilson, Inorg. Chem., 2009, 49, 638-645.
"Supramolecular Architectures Based on Organometallic Half-sandwich Complexes", T. B. Rauchfuss, K. Severin, in Molecular Nanostructures, J. Atwood, J. Steed, eds. Wiley-VCH, 2008, 179-201.
"Homogeneous Catalytic Hydrogenation of Dioxygen: a Step Toward an Organometallic Fuel Cell?" Z. M. Heiden, T. B. Rauchfuss, 2007, 129, 14303-14310.
"Cyanometallate Cages with Exchangeable Terminal Ligands", J. L. Boyer, H. Yao, M. L. Kuhlman, T. B. Rauchfuss, S. R. Wilson, Eur. J. Inorg. Chem. (invited paper for 10th anniversary issue) 2007, 2721-2728.
"Evolution of Organo-cyanometallate Cages: Supramolecular Architectures and New Cs+-specific Receptors", J.L. Boyer, M.L. Kuhlman and T.B. Rauchfuss, Accounts of Chemical Research, 2007, 40, 233-242.
"Redox-Switched Complexation/Decomplexation of K+ and Cs+ by Molecular Cyanometalate Boxes", J. L. Boyer, M. Ramesh, H. Yao, T. B. Rauchfuss, S. R. Wilson, J. Am. Chem. Soc. 2007, 129, 1931-1936.
"Proton-induced Lewis Acidity of Unsaturated Iridium Amides", Z. M. Heiden and T. B. Rauchfuss, J. Am. Chem. Soc. 2006, 128, 13048-13049.
"Competing H-H, S-S, and M-M Bond Formation in [Ru4S3(arene)4]z Clusters" M. L. Kuhlman, T. B. Rauchfuss, Angew. Chem. Int. Ed. 2004, 43, 6742-6745.
"Synthesis and Characterization
of the Hexagonal Prismatic Cage {THF![]() [PhB(CN)3]6[Cp*Rh]6}6+",
M. L. Kuhlman, H. Yao, T. B. Rauchfuss, Chem. Commun. 2004,
1370-1.
[PhB(CN)3]6[Cp*Rh]6}6+",
M. L. Kuhlman, H. Yao, T. B. Rauchfuss, Chem. Commun. 2004,
1370-1.
"Hybrid Cluster-Cages Formed
via Cyanometallate Condensation: Cs![]() Co4Ru6S2(CN)12,
Co4Ru9S6(CN)9, and Rh4Ru9S6(CN)9
Frameworks", M. L. Kuhlman, T. B. Rauchfuss, Inorg. Chem.
2004, 43, 430-435.
Co4Ru6S2(CN)12,
Co4Ru9S6(CN)9, and Rh4Ru9S6(CN)9
Frameworks", M. L. Kuhlman, T. B. Rauchfuss, Inorg. Chem.
2004, 43, 430-435.
"Research on Soluble Metal Sulfides: From Polysulfido Complexes to Functional Models for the Hydrogenases" T. B. Rauchfuss, Inorg. Chem. 2004, 43, 14-26.
